| Parasite | Anisakis simplex |
|---|---|
| Taxonomy | Nematoda, Secernentea, Anisakidae |
| Hosts | Chub mackerel (Scomber japonicus), Alaska pollack (Theragra chalcogramma), Pacific cod (Gadus macrocephalus), Chum salmon (Oncorhynchus keta), Japanese common squid (Todarodes pacificus), and many other marine fishes. |
| Infection site | Body cavity, visceral organs, muscle |
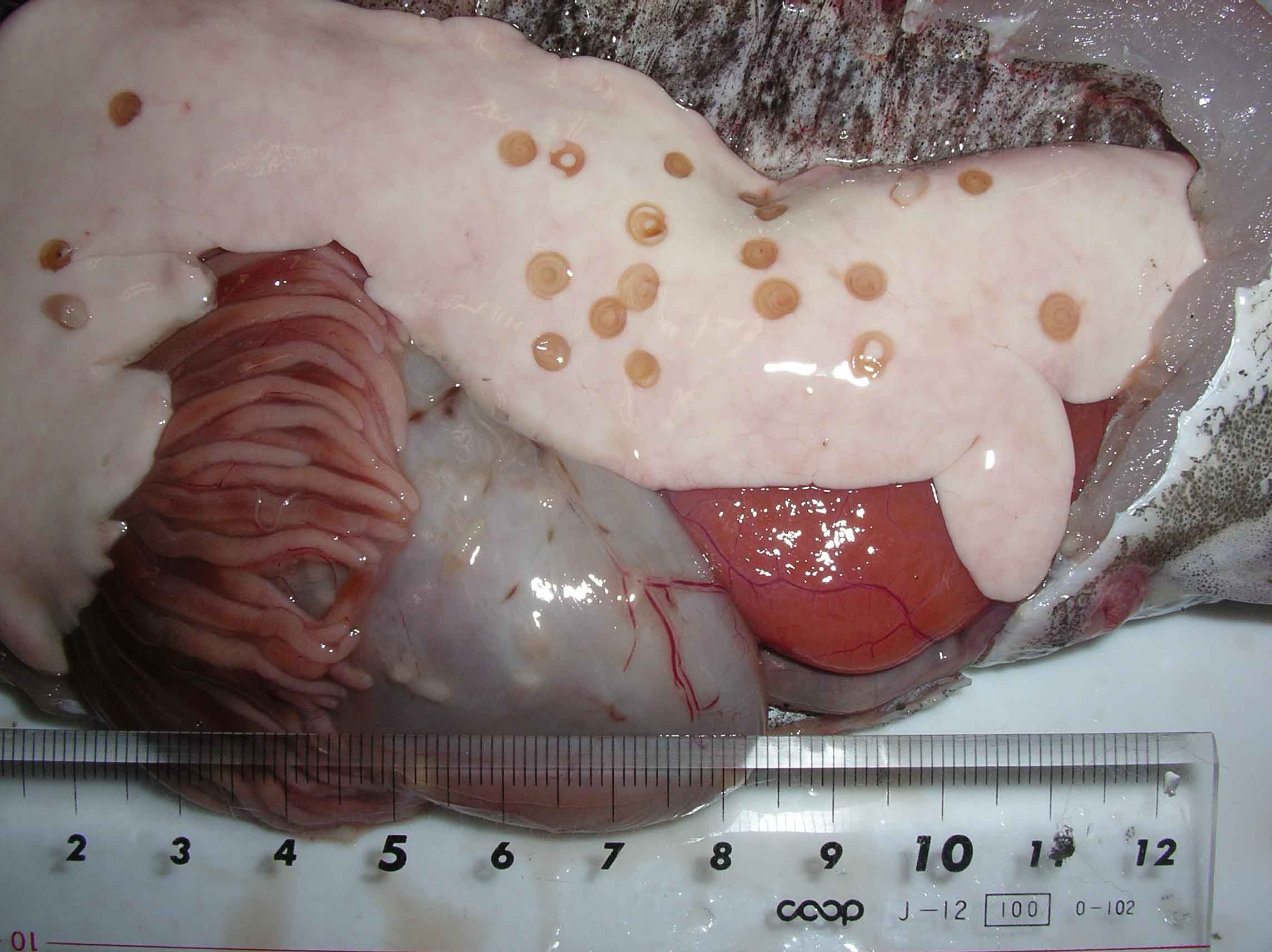
| Clinical signs | Infected fish exhibits no external abnormalities. A coiled filiform parasite is observed in the body cavity and on the liver or stomach (Fig. 1). In some fishes, this parasite infects the muscle (Fig. 2). |
| Parasitology | Final host is mainly whales, and the parasite develops to the adult inside their stomachs followed by the egg production. Released larva hatched from the egg is ingested by crustacean, e. g. euphausiid. Fishes are infected by eating such krill. Previously, it was considered that Anisakis type I, of which L3 larva causes anisakiasis, is only Anisakis simplex. However, recent studies revealed that type I is composed of A. simplex, A. simplex C, A. pegreffii, A. typical and A. ziphidarum (Mattiucci et al., 2002). In a limited sense (sensu stricto), eA. simplexf means only the one species A. simplex, meanwhile, in the broad sense (sensu lato), eA. simplexf includes A. simplex C and A. pegreffii. |
| Pathology | Pathogenicity to fish is considered to be low. |
| Health hazard | Live parasite (Fig. 3), a causative agent of anisakiasis, penetrates into the gut wall of human and causes the acute gastroenteritis, viz. violent abdominal pain, nausea and vomiting. Therefore, be careful when the host fishes is consumed raw. Heating or freezing to -20 C for 24 hrs is effective for killing the parasite. eRuibe (defrozen salmon sashimi)f is a traditional dish in Hokkaido, a northern part of Japanto, which seems to be effective to kill the parasite. The parasite elicits some patients allergic reactions such as urticaria (Del Pozo et al., 1997). The patient sometimes develops anisakiasis even after eating the processed foods since the allergens remain in such products. |
| Diagnosis | L3 larva of Anisakis type I has boring tooth at the anterior end (Fig. 4), oesophagous, ventriculus (Fig. 5) and intestine but no caecum. The larva of type I is distinguished from that of type II by the presence of the mucro (tail spine) and the blunt posterior end of type I (Fig. 6) (Koyama, 1974). However, larvae of type I cannot be classified further only by morphology. Therefore, sequences of rRNA or PCR-restriction fragment length polymorphism (RFLP) mapping are used to identify them (Abe et al., 2006; Valentini et al., 2006; Umehara, et al., 2006). |
| Other information | Anisakis simplex parasitizes over 200 species of wild fishes and squids. A. simplex is the most common species of anisakiasis in Japan. It is well known that Mr. Hisaya Mori, a Japanese famous actor, developed anisakiasis after eating eBattera (sushi of chub mackerel)f. |
| References | Abe, N., K. Tominaga
and I. Kimata (2006): Usefulness of PCR-restriction fragment length polymorphism
analysis of the internal transcribed spacer region of rDNA for identification
of Anisakis simplex complex. Japan. J. Infect. Dis., 59, 60-62. Del Pozo, M. D., M. Audicana, J. M. Diez, D. Munoz, I. J. Ansotegui, E. Fernandez, M. Garcia, M. Etxenagusia, M. Moneo and L. Fernandez de Corres (1997): Anisakis simplex, a relevant etiologic factor in acute urticaria. Allergy, 52, 576-579. Koyama, T (1974): I. Anisakidae larvae. 1. Morphology and classification. Fish and anisakis (The Japanese Society of Fisheries Science), Koseisha koseikaku, pp.9-19. Mattiucci, S., L. Paggi, G. Nascetti, C. P. Santos, G. Costa, A. P. Di Beneditto, R. Ramos, M. Argyrou, R. Cianchi and L. Bullini (2002): Genetic markers in the study of Anisakis typica (Diesing, 1860): larval identification and genetic relationships with other species of Anisakis Dujardin, 1845 (Nematoda: Anisakidae). System. Parasitol., 51, 159-170. Umehara, A., Y. Kawakami, T. Matsui, J. Araki and A. Uchida (2006): Molecular identification of Anisakis simplex sensu stricto and Anisakis pegreffii (Nematoda : Anisakidae) from fish and cetacean in Japanese waters. Prasitol. Int., 55, 267-271. Valenti, A., S. Mattiucci, P. Bondanelli, S. C. Webb, A. A. Mignucci-Giannone, M. M. Colom-Llavina and G. Nascetti (2006): Genetic relationships among Anisakis species (Nematoda: Anisakidae) inferred from mitochondrial COX2 sequences, and comparison with allozyme data. J. Parasitol., 92, 156-166. |
Fig. 6. Anus (arrowhead) and the mucro (arrow) of the posterior end of A. simplex.
(Photo by S. Urawa (2))
Fig. 5. A ventriculus (arrow) of A. simplex.
Fig. 4. A boring tooth (arrow) at the anterior end of the worm.
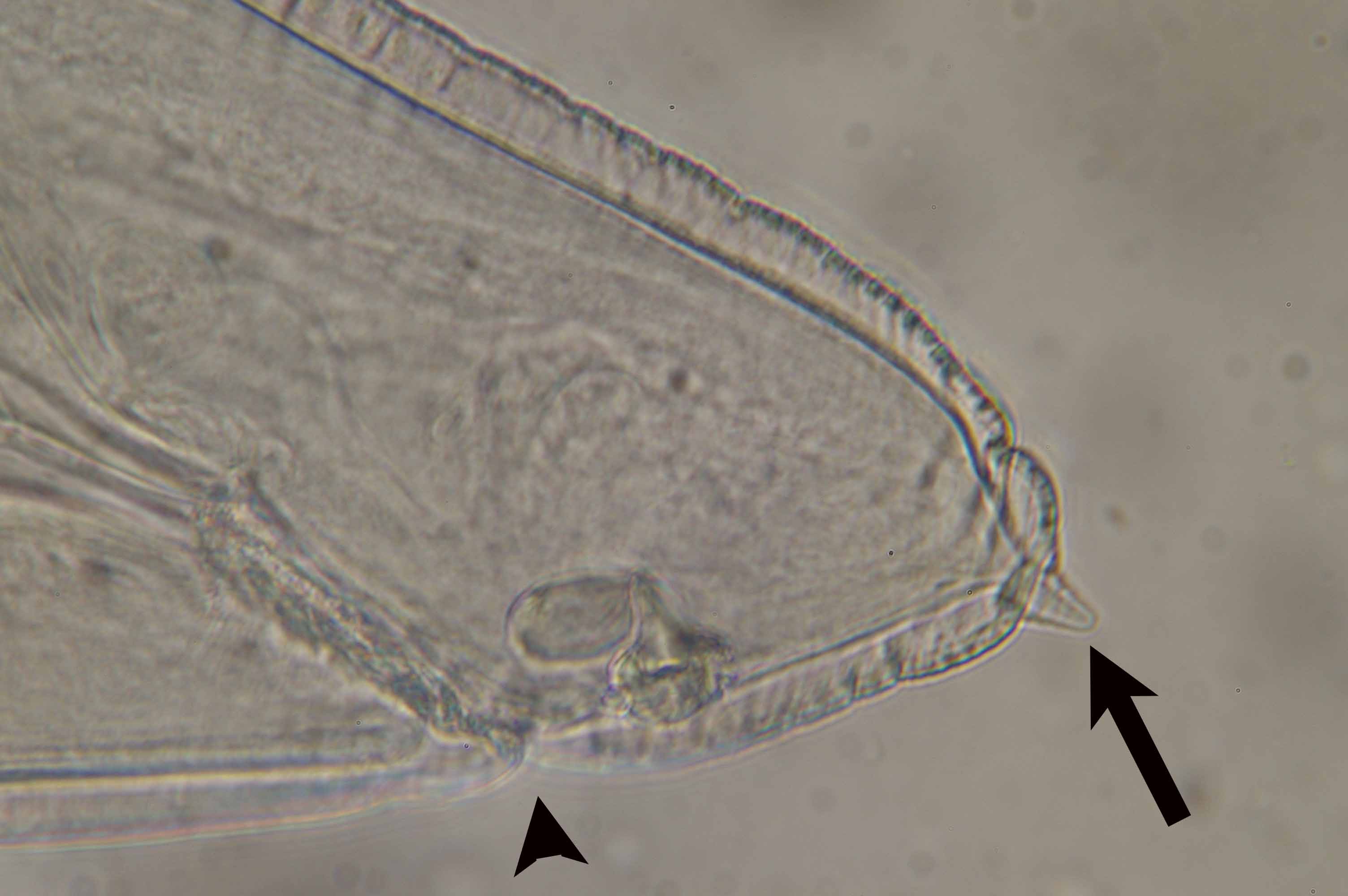
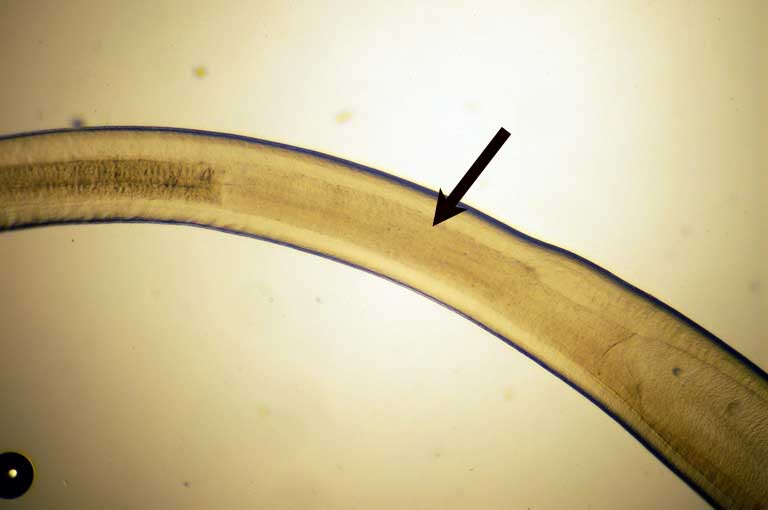
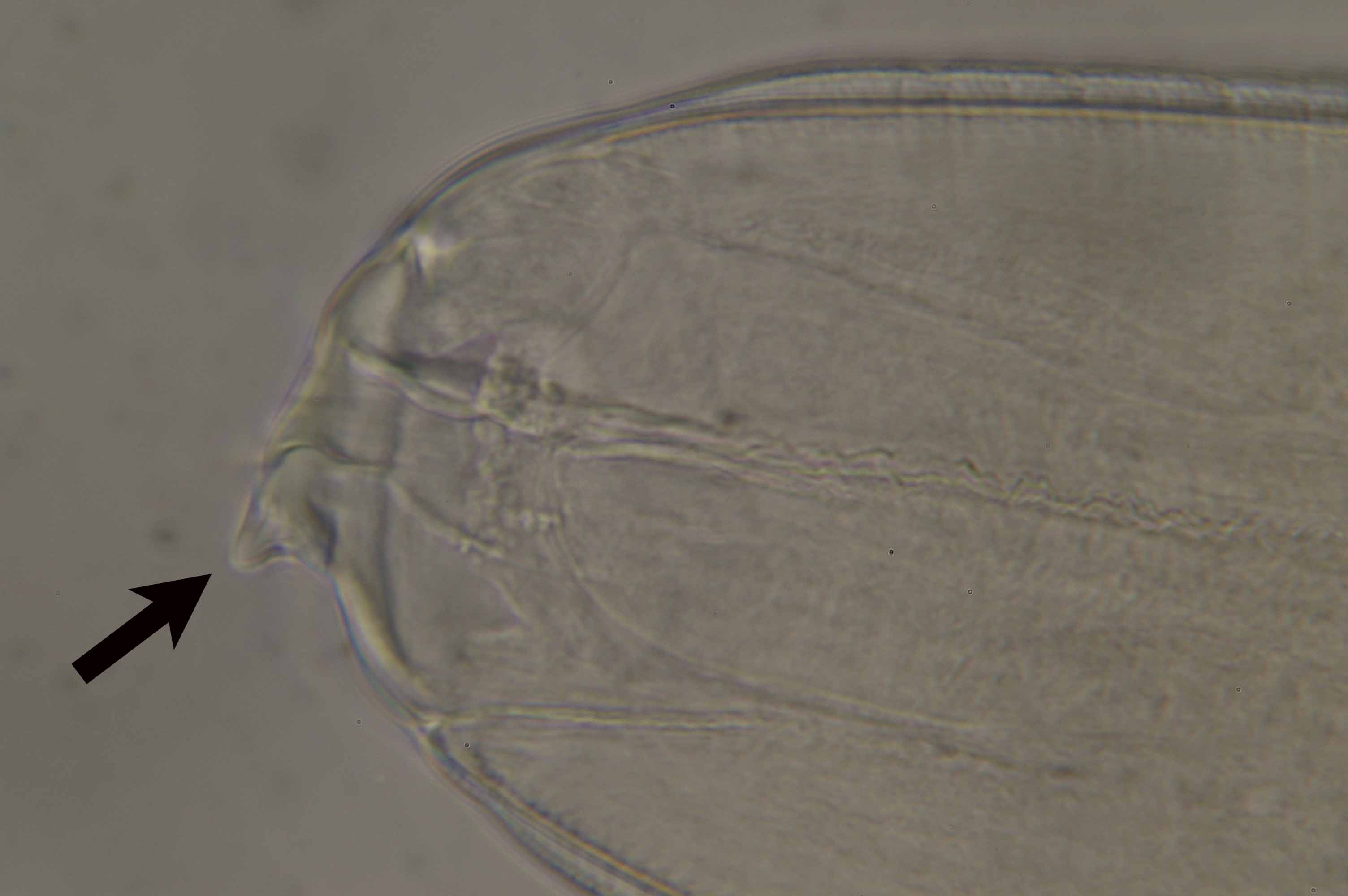
Fig. 3. Numerous live Anisakis from Alaska Pollack
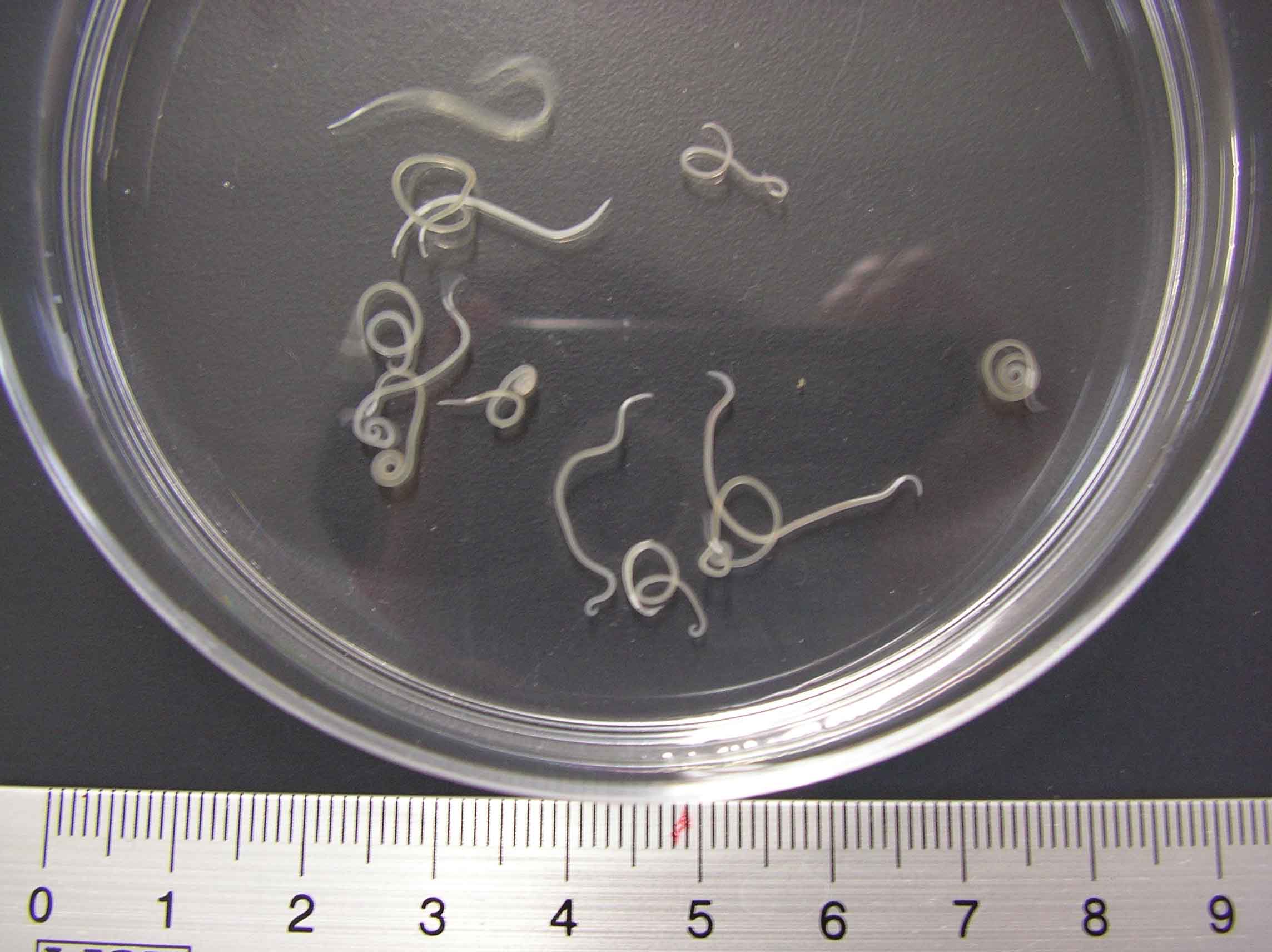
Fig.. 2. A. simplex in the muscle of chum salmon.
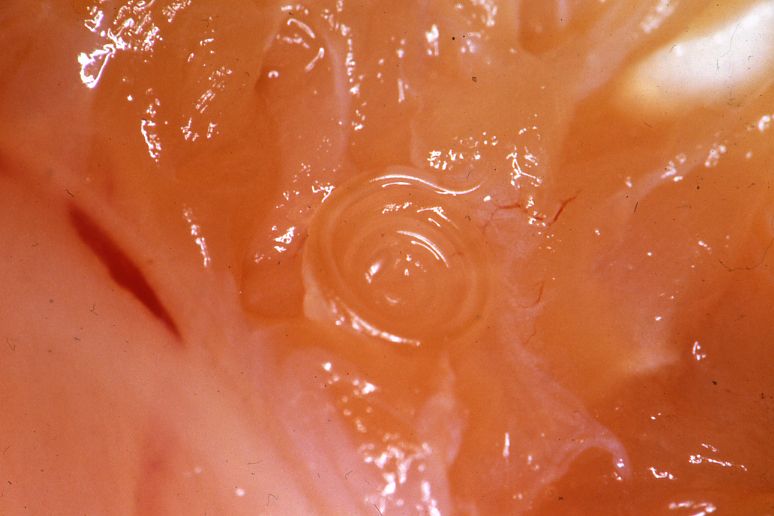
Fig. 1. Anisakis simplex on the liver of Alaska pollack